Particles in motion
Here is a great summary of what we will look at in section. The lady who speaks is not very interesting, but he explanations are clear and appropriate for the grade 9 level.
Essentially, what you need to know is that molecules move faster and faster the more energy they have (such as when they are being heated). And the faster they move, the further apart the individual molecules are.
Let’s get into the different states of matter. I’m sure you have seen the following chart before, so this should all be familiar.
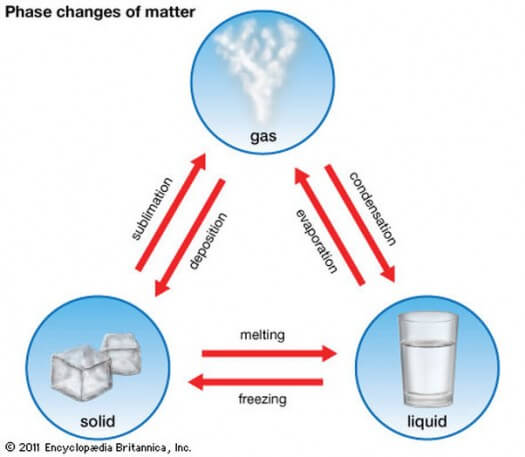
Now, let’s look at what this means at the molecular level!
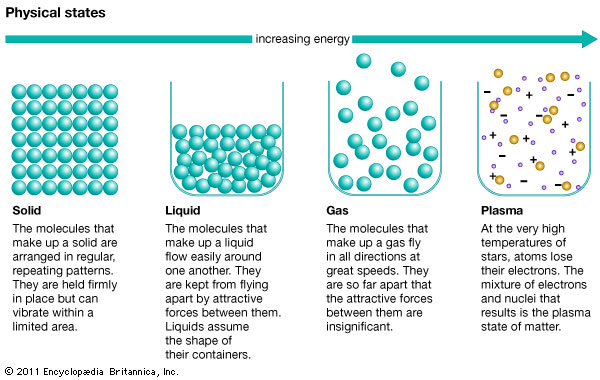
Note: the section on plasma is for enrichment only.
What you notice from the above graph is that the molecules represented by the green balls become less and less organized as you add energy (heat) to them and they move faster and go further and further apart.
Let’s talk about density just briefly.
Density describes the “pactness” of a substance, or in other words, how close together the molecules of a substance is. So knowing this, I have a question that I want you to think about. Let’s compare cold air and hot air. Using what you know about how molecules move with relation to temperature, which (cold air or hot air) would you expect to be more dense?
What’s important to know here is that molecules move faster when they are heated. The faster they move the more they bump into each other and spread apart. The farther apart the molecules are, the less dense they are. So, to answer the question above, cold air is going to be more dense and hot air will be less dense.
And as you know, when you have two substances with different densities, the one with the lower density will float up above the substance with a higher density. This is why you’ve heard the expression “hot air moves up.” So let me ask you one final question: Can you figure out how a hot air ballon works?